This site is supported by our readers. We may earn a commission, at no cost to you, if you purchase through links.
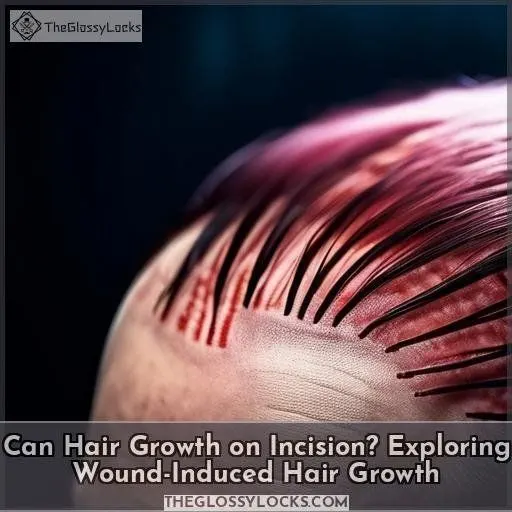 Yes, hair growth on incisions is possible, a phenomenon known as wound-induced hair growth. When skin sustains an injury, hair follicles can become activated, leading to excessive hair growth around the incision site.
Yes, hair growth on incisions is possible, a phenomenon known as wound-induced hair growth. When skin sustains an injury, hair follicles can become activated, leading to excessive hair growth around the incision site.
This occurs due to the body’s natural wound-healing processes and the release of specific growth factors that stimulate follicles. While it may seem concerning, it’s generally harmless and temporary. However, if the hair growth persists or causes discomfort, you should consult a healthcare professional.
Let’s explore the underlying mechanisms and potential management strategies in more detail.
Table Of Contents
- Key Takeaways
- Can Hair Growth on Incision?
- Hair Follicle Complications
- Donor Area Complications
- Recipient Area Complications
- General Complications
- Nonsurgical Complications
- Hair Follicles and Wound Healing
- Wound-Induced Hair Growth
- Androgenetic Alopecia
- Microneedling and Hair Growth
- Hypertrichosis
- Frequently Asked Questions (FAQs)
- What are the most common complications of hair restoration surgery?
- How does anesthesia affect hair growth after surgery?
- What is the relationship between wound healing and hair regeneration?
- What are the potential complications of microneedling for hair growth?
- How does hypertrichosis affect the outcome of hair restoration surgery?
- Conclusion
Key Takeaways
- Wound-induced hair growth is a phenomenon that can occur after bone fractures, burn injuries, and chronic venous ulcers.
- This condition is generally considered benign and temporary, resolving within a few months.
- The pathophysiology of wound-induced hair growth is not fully understood but may be related to the wound healing response and the role of hair follicles in this process.
- Understanding the relationship between wound healing and hair regeneration could potentially lead to better management of alopecia in the future.
Can Hair Growth on Incision?
Yes, hair growth can occur on incisions. Wound-induced hair neogenesis (WIHN) is a phenomenon where skin, sebaceous glands, and hair follicles regenerate following the occurrence of large, full-thickness wounds in adult mice, as well as in rabbits, sheep, and spiny mice.
This process is believed to recapitulate embryogenesis and provides a potential therapeutic window for manipulating cell fate during wound healing. However, not all wounds exhibit WIHN, as it only occurs in large wounds (>1 cm2), and hair follicles are only found in the center of these large wounds, not in the periphery.
Hair Follicle Complications
Hair follicle complications can arise from various factors, including the type of hair transplant procedure and the patient’s post-operative care. FUT (Follicular Unit Transplantation) and FUE (Follicular Unit Extraction) are two common hair transplant techniques with their own set of complications.
FUT involves cutting a strip of skin from the back of the scalp and then removing individual follicles. Complications include bleeding, infection, wound dehiscence or necrosis, numbness, and scarring. FUE, on the other hand, involves removing individual hair follicles for transplantation. Complications include donor site depletion, hypopigmentation, acute effluvium, buried grafts, and a higher transection rate.
Infections can occur when microbes enter open wounds at the site of the implanted hair follicles or at the reception site. Symptoms of an infected hair transplant include pus-filled abscesses, oozing pus, redness or discoloration, swelling, pain, itchiness, burning, bleeding, and warmth. Infections can lead to systemic symptoms such as fever, lethargy, swollen lymph nodes, headaches, nausea, and vomiting.
Folliculitis, an inflammation of the hair follicles, is a common complication after hair transplant surgery. It can manifest as red or discolored bumps that resemble acne and may occur weeks or months after the procedure. In most cases, no specific bacteria is identified, and the condition is known as sterile folliculitis.
To minimize the risk of complications, it’s essential to have your procedure at a licensed clinic that follows good hygiene habits, avoid picking at scabs or touching wounds, go to scheduled follow-up appointments, follow pre- and post-care instructions, and avoid alcohol and tobacco while recovering.
Donor Area Complications
Donor area complications in hair transplant procedures can include bleeding, folliculitis, infection, wound dehiscence or necrosis, and numbness.
These complications can occur due to various factors, including the surgical technique, anesthesia, stress, and positional alopecia.
Bleeding is a common issue during hair transplant surgery, but skilled surgeons and proper surgical techniques can minimize blood loss.
Folliculitis is an inflammation of the follicles and can be caused by an infection or an allergic reaction to the procedure.
Infection is another risk factor, especially in patients with a weakened immune system or poor wound care.
Wound dehiscence or necrosis can occur when the wound fails to heal properly, often due to factors such as advanced age, chronic corticosteroid use, malnutrition, systemic diseases like diabetes, or tension in wound closure.
Numbness is a rare complication but can occur due to nerve damage during the procedure.
Scarring is also a concern, with FUT (Follicular Unit Transplantation) leaving a linear scar and FUE (Follicular Unit Extraction) causing pinpoint scars.
Recipient Area Complications
Recipient area complications in hair transplant surgery can lead to an unnatural appearance, which is a common concern for patients. These complications can include poor hairline, low density, and edema. In some cases, necrosis and postoperative folliculitis or pustules may occur. These issues can be distressing for patients and may require additional treatments or follow-up care.
One of the complications that can affect the recipient area is poor hairline design, which can lead to an unnatural appearance. This can be due to inadequate planning or execution during the procedure. Another issue is low density, which can result in an uneven or thin appearance. This can be caused by inadequate graft placement or insufficient graft survival.
Edema, or swelling, is another common complication in the recipient area. This can occur due to fluid accumulation and can cause discomfort and a stretched appearance. In some cases, necrosis may occur, which is the death of skin tissue. This can be caused by poor blood flow or infection. Postoperative folliculitis or pustules can also develop, which are infections of the hair follicles. These complications can be managed with appropriate care and treatments, but they can still be distressing for patients.
To minimize the risk of recipient area complications, it’s essential to follow proper surgical techniques and patient care. This includes careful planning and execution of the hairline design, ensuring adequate graft placement, and monitoring for signs of infection or poor wound healing. Additionally, proper postoperative care, such as wound care and medication management, can help to reduce the risk of complications.
General Complications
Hair transplant surgery is a complex procedure that involves numerous potential complications.
- Anesthesia complications: Adverse reactions to local anesthetics can occur, including systemic reactions that may impact the patient’s social and psychological well-being.
- Infection risk: While the risk of infection is low, it’s still a concern, especially in the donor and recipient sites.
- Pain management: Proper pain management is crucial, with patients typically requiring 2-3 pain relievers on the first evening after surgery.
- Donor site damage: This can include complications such as bleeding, numbness, and wound dehiscence or necrosis, which can affect the quality of the hair grafts.
In addition to these general complications, hair transplant surgery also involves specific complications for the donor and recipient areas, as well as nonsurgical complications related to microneedling, protein deficiencies, and medication side effects. It’s essential for patients to be aware of these potential complications and for surgeons to be well-versed in their prevention and management.
Nonsurgical Complications
Hair transplantation is a cosmetic surgery, and any complication can significantly impact the patient’s cosmetic and psychological outcome.
- Patient dissatisfaction: This can occur due to poor diagnosis of the reason for hair loss, a medical condition that the transplant can’t address, or unrealistic patient expectations. Patients should be counseled about the limitations of hair transplantation and the importance of realistic goals.
- Drug-induced complications: Certain medications, such as corticosteroid injections, can cause complications like hypopigmentation or hyperpigmentation of the scar and nearby areas of the donor site.
- Keloid and hypertrophic scar: These are rare complications in hair transplantation. If they persist for a longer period and divert the patient’s attention, local application of steroid ointment and moistening lotions may be needed.
- Donor hair effluvium: This can occur due to the stress of the transplant procedure, causing existing hair to fall out in the donor area.
- Hiccups: Although rare, hiccups can be a complication of hair transplantation.
To minimize these complications, proper surgical technique, wound care, and counseling before surgery are essential. Every patient should be individually planned and operated with an aim to zero-down the complications and complaints.
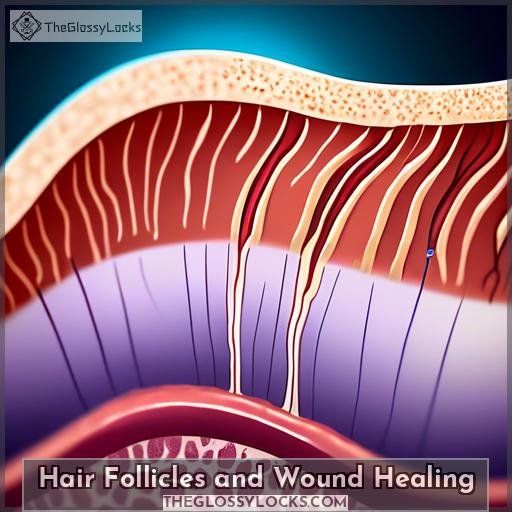
Hair Follicles and Wound Healing
Hair follicles play a crucial role in wound healing, as they’re the main anatomical structure that contributes to the healing process of cutaneous wounds. Wound healing starts around the hair follicles, with both the epithelial portion and the mesenchymal portion of the hair follicle contributing to the healing of skin wounds. The granulation tissue that regenerates comes from the connective tissue surrounding the hair follicles, and wounds in hairy areas heal faster than in non-hairy areas. The hair follicle is the main repository of cutaneous stem cells with multipotent capacity, and at the cellular level, follicular stem cells are key players in the proliferation and differentiation of follicular cells in the wound bed.
Hair follicles can also regenerate after removal of superficial layers of the skin, as observed by G.H. Bishop in his study published in the American Journal of Anatomy. The wound healing process involves the reepithelialization of the wound starting around the remaining hair follicles, and when the skin is destroyed down to the deep dermis, reepithelialization starts around the hair follicles.
In the realm of hair regeneration, laser therapy has been designed to enhance and stimulate hair growth by improving blood circulation and promoting the repair and regeneration of tissue. Low-level laser therapy (LLLT) has been found to stimulate hair growth by triggering vasodilation and potentially causing hair follicles to enter the anagen (growth) phase of the hair growth cycle. This mechanism of action is similar to minoxidil, one of the medications approved by the FDA to treat hair loss.
Hair follicle regeneration relies on hair follicle stem cells (HFSCs), and understanding the signals in controlling survival, proliferation, and differentiation of HFSCs is crucial for hair follicle regeneration and skin wound healing. Hair follicle transplantation has been explored for wound repair, and the results indicate that it can promote skin regeneration and wound healing.
Wound-Induced Hair Growth
Wound-induced hair growth is a fascinating phenomenon that has been reported in cases of bone fractures, burn injury, and chronic venous ulcers. This process, also known as wound-induced hair neogenesis, isn’t fully understood, but it’s believed to be related to the wound healing response and the role of hair follicles in this process.
- Localization: The hair growth can be localized, affecting a specific area around the wound, such as the knee and leg in a case of a TKA (total knee arthroplasty) wound.
- Pathophysiology: The pathophysiology of this phenomenon is still being investigated, but it’s thought to be related to the wound healing response and the role of hair follicles in this process.
- Prognosis: Wound-induced hair growth is generally considered a benign, temporary condition that resolves spontaneously within a few months. Physicians should reassure patients of its transient nature.
Understanding the relationship between wound healing and hair regeneration could potentially lead to better management of alopecia (hair loss) in the future.
Androgenetic Alopecia
Shifting gears from the curious phenomenon of wound-induced hair growth, let’s tackle the head-scratcher that’s androgenetic alopecia (AGA).
This common form of hair loss can really throw a wrench in your self-image, as it stealthily progresses, leaving behind a trail of hair miniaturization.
But don’t throw in the towel just yet!
The quest for luscious locks isn’t a lost cause.
Alopecia treatments are evolving, with microneedling effects showing promise in regulating hair growth.
Imagine tiny needles rolling over your scalp, reigniting those sleepy follicles.
It’s like a wake-up call for your hair, potentially putting the brakes on AGA progression and steering you back to a fuller head of hair.
Microneedling and Hair Growth
Microneedling has emerged as a versatile and effective cosmetic procedure with applications in hair restoration, skin rejuvenation, and improving acne scars. This minimally invasive treatment involves the use of a skin roller with tiny needles that create micro-injuries in the skin, triggering a wound-healing response that can stimulate hair growth. Microneedling is particularly effective when combined with other treatments, such as platelet-rich plasma (PRP) therapy or minoxidil, which can enhance the effects of microneedling and promote hair regrowth.
When it comes to hair loss, microneedling can be beneficial for individuals with androgenetic alopecia (AGA) and some cases of alopecia areata. The treatment works by increasing blood flow to the scalp, providing essential nutrients to hair follicles, and stimulating the production of collagen and growth factors, which can help improve hair density and overall hair health.
It is essential to note that microneedling should be performed by a qualified healthcare professional to ensure safety and effectiveness. At-home microneedling devices with short needles may not have adverse side effects, but repeated overuse can be harmful. Professional microneedling devices for hair loss are calibrated to puncture scalp skin more deeply, reaching the bulge region of hair follicles, where growth-boosting stem cells reside.
Hypertrichosis
Have you ever wondered if hair can grow on incisions? Well, the answer is yes, and it’s a phenomenon known as hypertrichosis. This condition is characterized by excessive hair growth for an individual’s age, sex, or ethnicity. It can be generalized or localized, and its etiology can be congenital or acquired.
Here are some key points about hypertrichosis:
- Transient Nature: Postsurgical hypertrichosis is a benign, temporary condition that resolves spontaneously within months.
- Hair Regeneration: The pathophysiology of postsurgical hypertrichosis isn’t fully understood, but it may be related to wound healing and hair regeneration.
- Alopecia Management: Understanding the relationship between wound healing and hair regeneration could potentially lead to better management of alopecia.
- Uncommon Complication: Hypertrichosis is an uncommon complication of surgery, but it’s essential for physicians to reassure patients of its transient nature.
In the realm of hair follicles, hypertrichosis isn’t only a complication but also a potential key to unlocking secrets about hair growth and wound healing. As we navigate the complexities of hair regeneration, it’s not merely about understanding the pathophysiology, but also about designing treatments that are tailored towards enhancing our understanding of this ever-evolving field.
Frequently Asked Questions (FAQs)
What are the most common complications of hair restoration surgery?
The main complications? Poor hairline design, unnatural density, edema, necrosis, infection, anesthesia reactions, and dissatisfaction. Proper planning and technique minimize risks for this cosmetic procedure.
How does anesthesia affect hair growth after surgery?
You won’t experience significant hair growth after surgery due to anesthesia. However, the wound-healing process may temporarily stimulate follicles, leading to localized excessive hair growth that resolves spontaneously.
What is the relationship between wound healing and hair regeneration?
Interestingly, wound healing can trigger hair regeneration. When skin is injured, follicles become activated, promoting new hair growth – a phenomenon researchers are exploring to treat hair loss.
What are the potential complications of microneedling for hair growth?
You’re in for potential pain, redness, bleeding, infection, scarring, or worsening hair loss from microneedling. But when done properly by pros, it can stimulate new growth for some folks battling thinning strands.
How does hypertrichosis affect the outcome of hair restoration surgery?
Picture a lush forest flourishing atop your surgical scar – that’s hypertrichosis post hair transplant. While transient, it signals healthy healing and revitalized follicles thriving anew.
Conclusion
Like a delicate tapestry woven from strands of expertise, understanding wound-induced hair growth requires meticulous attention to detail. While microneedling promotes follicle stimulation, hypertrichosis risk necessitates caution post-incision to prevent unwanted hair proliferation. Embracing evidence-based strategies tailored to your unique hair restoration journey ensures optimal outcomes. You’ll navigate complexities confidently, unlocking the secrets to revitalized tresses.